Culinary Foams Overview
Much of molecular gastronomy centers around the idea of changing textures, of presenting familiar flavors in unfamiliar presentations. Modernist chefs particularly like to use foams for their luscious textures and clean delivery of flavor.
Foams have been central to cooking long before the modernist movement. Both eggs and dairy cream contain natural emulsifiers that allow the creation of stable foams for both savory and sweet applications. But, the novel ingredients used in molecular gastronomy have given chefs the freedom to experiment; to create foams out of flavors previously thought impossible.
Although foams are often thought of as garnishes or toppings to dishes, many foams are the main attractions. Most ice creams are foams, as are meringues, mousses, and any baked good that rises, such as a brioche bun or a spongy cake.
As you can already probably see, foams have a myriad of applications. Using, choosing, and perfecting foams should be a key skill for any modernist cook.

What is a foam?
So what is a foam, exactly? And why can it take so many forms? By definition, a foam is simply any liquid or solid that has a gas suspended in it. A kitchen sponge is a foam of air suspended in solid fibers. The head on a beer is a foam of carbon dioxide and air suspended in liquid beer.

[image: gosheshe]
Take a look at the image above. Notice that the structure of the kitchen sponge looks a lot like the cross-section of a slice of bread. That's because bread is a foam of air and carbon dioxide (produced by yeast) suspended in solid flour and other ingredients.
Solid foams are easy to understand---the solid portions prevent suspended gas from escaping. Liquid-based foams require an additional ingredient: a surfactant.
Surfactants lower the surface tension of water and allow water and oil to mix. That's why soap is so effective against oils and stains: it gives water the ability to wash away the oil.

[image: Richard Taylor]
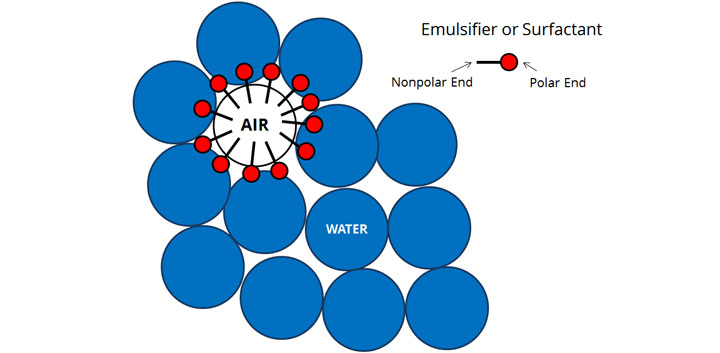
If you've ever washed dishes before, you've probably also noticed that a foam inevitably forms in the water. That's because the part of the soap molecule that bonds well with oil also bonds well with air. The result are tiny bubbles that stay stable for much longer than would happen with water alone. In this capacity, the surfactants in soap act as emulsifiers, molecules that help normally repulsive ingredients (like oil and water or water and air) to mix.
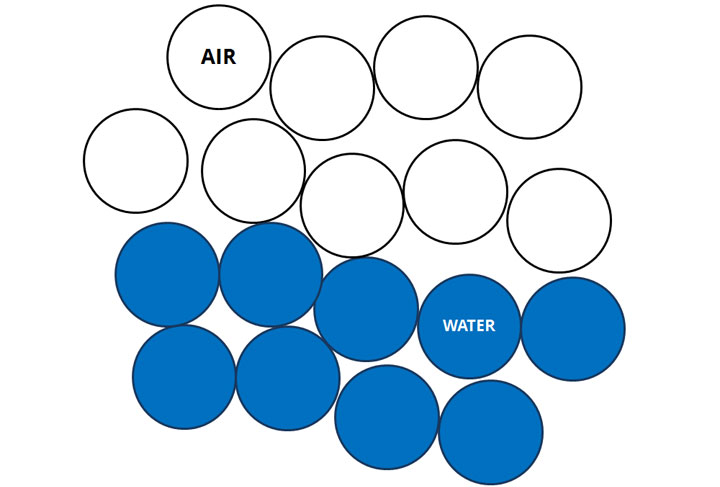
Without an emulsifier, water and air don't mix.
But emulsifiers/surfactants have both polar and nonpolar ends that attach to water and air, respectively. If we were able to look at the formation of each individual air bubble in a foam, it'd look something like the illustration above.
By Kevin Liu