Spherification Overview
The spherification technique was introduced by el Bulli in 2003. It consists of a controlled jellification of a liquid which forms spheres when submerged in a bath. The spheres can be made of different sizes and have been given names like caviar when they are small, eggs, gnocchi and ravioli when they have larger size. The resulting spheres have a thin membrane and are filled with the flavored liquid. A slight pressure of the mouth on the sphere makes it burst and release an amazing explosion of flavor. The spheres are flexible and can be carefully manipulated. It is possible to introduce solid elements in the sphere which will remain in suspension in the liquid giving the possibility of introducing multiple flavors and textures in one preparation. (see spherification recipes).

-
$99.95
-
$179.95
-
$275.95
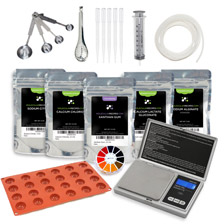
It sounds complicated but the spherification technique can be easily mastered at home with one of our Molecular Gastronomy kits and the detailed instructions on our website. The Molecular Gastronomy Essentials Kit has all the ingredients and tools necessary to make any type of sphere. If you are also interested in exploring other techniques, you may want to consider the Molecular Gastronomy Premium Kit or the Molecular Gastronomy Ultimate Kit.
There are two main kinds of spherification techniques and each of them has its advantages and disadvantages which make them more suitable for certain recipes. The Basic Spherification technique consists of submerging a liquid with sodium alginate in a bath of calcium. The Reverse Spherification technique consists of submerging a liquid with calcium content in a bath of sodium alginate. When the liquid drops into the bath, a thin coat of gel forms around the droplet as the calcium reacts with the sodium alginate.
There are also a couple of other techniques to create spheres which consist of instant jelling by immersing the liquid in cold oil or liquid nitrogen, but these are made with a completely different process and the resulting spheres are solid and have no liquid inside. So these are usually not considered "spherification" techniques.
Basic Spherification
The Basic Spherification technique is ideal for obtaining spheres with a very, very thin membrane that is almost imperceptible in your mouth. It results in a sphere that easily explodes in your mouth as if there is no solid substance between your palate and the liquid. The main problem of this technique is that once the sphere is removed from the calcium bath, the process of jellification continues even after rinsing the sphere with water. This means that the spheres need to be served immediately or they would convert into a compact gel ball with no magical liquid inside. The other issue of this technique is that jellification does not occur if the liquid acidity is high (PH<3.6) but this can be corrected by adding sodium citrate to the liquid to reduce the acidity level before the spherification process. The Basic Spherification technique doesn't work with ingredients that have high calcium content Examples of Basic Spherification are "Spherical Mango Ravioli" , "Liquid Pea Ravioli", "Caviar of Cointreau" (shown below). Learn more about Basic Spherification.
We cannot display this galleryReverse Spherification
The technique of Reverse Spherification is much more versatile than Basic Spherification as it can make spheres with almost any product. It is best for liquids with high calcium content or alcohol content. Contrary to the spheres made with the Basic Spherification process, these spheres have a thicker membrane and are long-lasting as the process of jellification can be stopped when the sphere is removed from the sodium alginate bath and rinsed with water. Thanks to these characteristics, the Reverse Spherification spheres can be manipulated more easily and can be used in more ways (for example as fillings in sponge cakes or mousses, in cocktails or can even be macerated in aromatized olive oil for a few days). Examples of Reverse Spherification are "Yoghurt Spheres", "Liquid Mozzarella Spheres" and "Spherical Olives" (shown below). Learn more about Reverse Spherification.
We cannot display this galleryThe Science of Spherification
Sodium Alginate is a natural polysaccharide product extracted from brown seaweed that grows in cold water regions. In presence of calcium, sodium alginate forms a gel without the need of heat.
In Basic Spherification, the gelling occurs thanks to the diffusion method in which the crosslinking calcium ion diffuses from an outer reservoir into an alginate solution. Gels form when a calcium salt is added to a solution of sodium alginate in water. The gel forms by chemical reaction, the calcium displaces the sodium from the alginate, holds the long alginate molecules together and a gel is the result. No heat is required and the gels do not melt when heated. The gel coating is formed inside the droplet. Because the calcium ions continue diffusing towards the center of the droplet even after removing the sphere from the calcium bath, the gelification process continues and will eventually form a solid gel sphere.
In Reverse Spherification, the calcium ions diffuse from the droplet into the alginate bath, forming a gel coat outside the droplet of flavored liquid. Because the calcium ions are diffusing from the inside out and no alginate molecules are getting into the droplet, the gelification process stops as soon as the spheres are removed from the alginate bath. This allows you to store the spheres for later use.





 (11 votes, average: 3.82)
(11 votes, average: 3.82)