Emulsions Overview
Any schoolchild is familiar with a simple rule of chemistry: oil and water don't mix. But modernist chefs are not ones to be slowed down by an immutable law of nature.
Emulsions, with their creamy textures and unique flavors, combine oil and water into a single stable mix. Depending on the thickener and emulsifier used, an emulsion can have textures ranging from milky to thick and creamy.
Many emulsions exist outside of molecular gastronomy. Eggs are natural emulsions. All vinaigrettes are an emulsion. Milk and dairy products are emulsions. Ice cream, mayonnaise and chocolate are emulsions. Many of the emulsions used in molecular gastronomy use ingredients that have long been available, but it is the creativity behind modernist creations that define them as innovative. There are also modernist emulsifiers that allow you to make more stable emulsions and emulsions without dairy or eggs.

What is an emulsion?
Emulsions are closely related to foams. Whereas foams are a dispersion of gas in a liquid or a solid, emulsions are dispersions of one liquid in another liquid.
Normally, oil and water don't mix. This is because they repel each other due to differences in electrical charges in their molecules. Water molecules are electrically unbalanced or polar and they attract each other. Oil molecules are non-polar and therefore don't play well with water molecules.
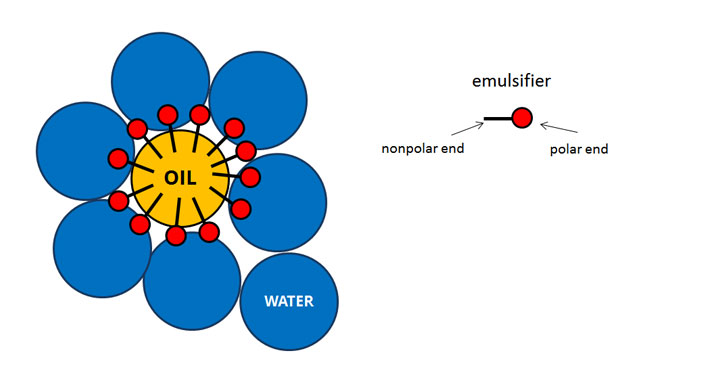
When you add emulsifiers to oil and water, they gain the ability to mix. Surfactants are the most widely type of emulsifier used in modernist cuisine. That's because these emulsifiers have the ability to attach to both water and to oil to form an emulsion. A simplified way of looking at this is to say than an emulsifier has both polar and nonpolar ends. Or you might say that one part of the molecule is hydrophillic (water-loving) while the other side is hydrophobic (water-fearing). In reality, naturally-occurring emulsifiers are usually groups of different molecules that work in concert, but the end result is the same.
You may be surprised by this but soap is a surfactant and cleans by emulsification. The emulsifying properties of soap create an emulsion of the grease with the water that is then washed away.
By Kevin Liu