Emulsion Types
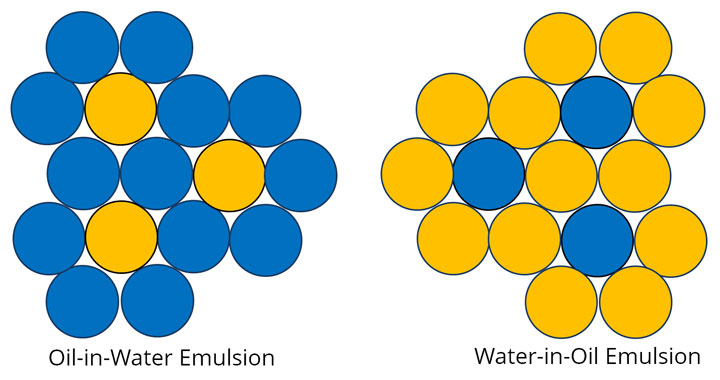
There are two basic types of emulsions: oil-in-water (O/W) and water-in-oil (W/O). These emulsions are exactly what they sound like, as pictured below.
In every emulsion there is a continuous phase that suspends the droplets of the other element which is called the dispersed phase. In an oil-in-water emulsion, the continuous phase is the water and the dispersed phase is the oil while in a water-in-oil emulsion the oil is the continuous phase.
Counterintuitively, the type of emulsion does not depend on the actual amounts of oil and water present in an emulsion. For example, vinaigrettes are oil-in-water emulsions even though there is more oil in a vinaigrette than water (vinegar).
For most recipes, it doesn't matter what type of emulsion you've created, as the end result is the same. The major exception is with dairy. Consider the difference between cream and butter:
Cream and butter are literally the same thing. To make butter, you simply mix cream until the emulsion reverses; that is, it transforms from a oil-in-water emulsion into a water-in-oil emulsion. But despite this being the only difference between cream and butter, the effect on taste and texture are significant.
Most emulsions will not turn into butter even if you whip them for a long time, but some emulsions can break down with too much stirring. For example, a mayonnaise will not form correctly if it is over-beaten or the oil is incorporated too quickly.
It is important to know the type of emulsion when adding an emulsifier as the preparation process changes but we'll explain this later.
How to make an emulsion
Mechanical Force - To make an emulsion you first need to apply a mechanical force to break down the dispersed phase into small droplets that become suspended in the continuous phase. For this you can use a whisk, blender or other lab equipment such as rotor-stator homogenizer. We'll cover these later.
Emulsifier - The next problem to solve is to make the emulsion stable. The higher the force you applied when making the emulsion, the smaller the droplets and the more stable the emulsion is. However, no matter how small the droplets are, the ingredients will eventually separate without the presence of an emulsifier that keeps the molecules with different polarity from repelling each other.
Thickener - Finally, adding a thickener to the continuous phase can make the emulsion even more stable as this makes it more difficult for the dispersed droplets to move and combine.
Any mixture of oil and water can be transformed into an emulsion, even without emulsifiers. However, without emulsifiers, the mixture will also quickly separate back into its immiscible (not mixable) parts. You can try a simple experiment with oil and water. Quickly whisk together oil and water, and it will eventually turn into an cloudy mixture. You'll notice plenty of bubbles, however, and those bubbles will quickly grow larger until the water and oil are completely separate.

[image: Nick Kean]
If, however, you use the emulsifier mustard and thickening agents like honey or xanthan gum, it's feasible to mix a stable vinaigrette by hand. The dressing may separate over time, but will likely stay emulsified through a dinner service.
Properties of an emulsion
Viscosity
When you mix oil and water, the resulting emulsion usually has a higher viscosity than each of the ingredients before the emulsification process. This effect is produced by the interaction of the molecules in the emulsion. For example, mayonnaise is more viscous than the oil and lemon juice it is made of. Most emulsions are shear-thining fluids which means that the viscosity decreases if you start stirring them strongly.
Color
The transparency and color of the emulsion depend on the size of the droplets of the dispersed ingredient. The smaller they are, the whiter the color of the emulsion. This is due to the way the droplet size affects the light reflection.




 (5 votes, average: 4.40)
(5 votes, average: 4.40)