Gels Overview
In physics, it takes either a large amount of energy or a significant change in pressure to transform a liquid into a solid. Modernist chefs achieve this monumental feat with just a few sprinkles of powder to form a gel! In this class we'll explain everything you need to know about gelification to create your own innovative gels.
You've probably had flavored gelatin growing up. The jiggly, bouncy dessert delights with its combination of fun texture and sweet flavor. As it turns out, gelatin is not the only powder that gels.
A group of ingredients known as "hydrocolloids" can create a gel from a wide variety of ingredients and are designed for specific applications. The term "hydrocolloid" covers a wide range of materials. Technically, a hydrocolloid is any molecule that can be evenly dispersed through water. We generally mean the term to describe ingredients that have either a thickening or gelling effect. The difference between a thickened liquid and a gel? A gel can display the characteristics of a solid, while a thickened liquid always behaves as a liquid.
These modernist ingredients allow molecular gastronomy chefs to make innovative gels such as a hot gel, a gel that forms when heated and melts when cooled, a fluid gel, a gel that can be sculpted, a gel sphere with liquid inside, gel beads, a gel spaghetti, crispy films from dehydrated gel, coating gels for solid ingredients and many other creative gels that will impress your diners.

What is a gel?
A gel is a jelly-like substance that can have properties ranging from elastic and soft to brittle and stiff. Gels are mostly composed of liquid but they behave more like a solid thanks to a three-dimensional cross-linked network within the liquid. The characteristics of this crossed-linked network determine the properties of the gel. So in short, a gel is a dispersed liquid in a continuous solid phase.
How does a gel work? We'll borrow some of the images and explanations we used to talk about thickeners. Every gelling agent is a thickener, though not all thickeners will form a gel.
Let's look at cornstarch, a common kitchen hydrocolloid, as an example.
When you buy a canister of cornstarch, it arrives in powdered form. The powder has very little body or thickness. At a microscopic level, what's happening is that the starch molecules from the corn are tightly wound together and dehydrated. Each packet of starch, represented below as spirals, stands alone and does not attach itself to any other packet of starch.
When you add cold water to cornstarch, the starch molecules evenly disperse into the water. In cooking, this is called a slurry. The packets of starch are still in their tight spirals, but now they are evenly spread into water.
[image: Rebecca Siegel]
When you heat cornstarch that has been dispersed in water, the heat and water act together to hydrate the cornstarch molecules. The tightly-wound spirals of starch unwind and begin to give a little thickness.
[image: maya83]
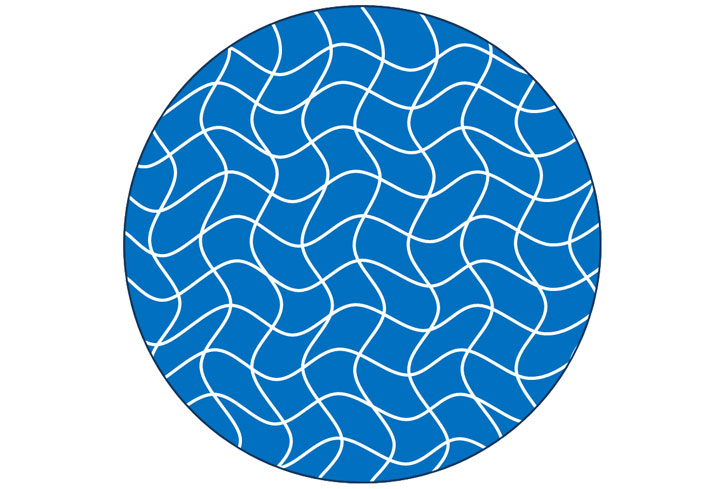
In the case of a gel, the long strands of polymers form a crosslinked network that holds its shape. Imagine the shape of a honeycomb, each cell of the honeycomb is an hexagon filled with honey. The majority of the mass of the comb is the liquid honey, but the whole is held together by the solid portions. (Honeycombs are not actually gels, but it's a helpful analogy).
In real life, the structures formed by a gel's hydrocolloid are far more complex than a simple hexagon. Below is a visual representation of how a gel organizes its shape.
By Kevin Liu




 (3 votes, average: 4.67)
(3 votes, average: 4.67)