Making Ice Cream Science
The science of ice cream is fascinating. There is so much going on with ice cream that there is plenty to learn and perfect. Frozen desserts are a solution, a suspension and a foam at the same time. Ice crystals are suspended in a thick syrup that also holds air bubbles. Let's take a look at the process of making ice cream and what is happening at the molecular level. There are 5 steps to making ice cream, they are: preparing the base, chilling, aging, freezing/churning and hardening. In this lesson, we will take a look at each step in detail.
 Preparing the Base
Preparing the Base
To prepare the base it is essential to combine the ingredients in appropriate amounts to make the ice cream. As we learned in the Traditional Frozen Treats lesson, most ice creams are 20-29% milk, cream or other dairy, 12-16% sweeteners, .2-.5% stabilizers, and 55-64% water which comes from the milk or other ingredients. In the lesson The Perfect Ice Cream Base we will explain how the different ingredients in the ice cream base affect the final result so you have the tools to troubleshoot, adjust and experiment with recipes as needed. We are also going to provide you with some foolproof ice cream base recipes to get you started.
Heating the Base
Pasteurization: Heating the ice cream base serves two purposes, pasteurization, and homogenization. When heating the ice cream base, you’ll first heat it to kill any bacteria. This is especially important when creating ice cream with an egg yolk emulsion, typical of Italian and French-style ice cream which have a custard-base. American-style ice cream, which does not contain eggs, is usually not heated but the base could benefit from it for the following reason.
Homogenization: The second purpose of heating ice cream is homogenization. Heating is not required for homogenization but when heating the base the results could be better. This is done by churning or stirring the ice cream while heating it. It helps break down and disperse the fat globules in the ice cream base and prepares the ice cream base for the aging and chilling steps. It prevents the fat from separating from the water in the mixture.
The ice cream base should be a stable oil in water emulsion before proceeding to the aging or chilling steps. At a molecular level, what is happening during the homogenization process is that the milk fat globules are stabilized by the milk protein casein micelle. In other words, the milk fat is being emulsified with the water (mostly coming from the milk) by the milk proteins.
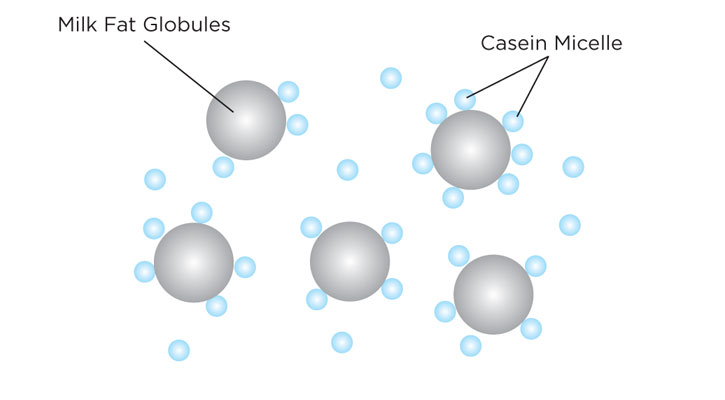
Stable oil in water emulsion by homogenization. Milk protein casein micelle stabilizes milk fat globules
Chilling
Chilling the ice cream base is important to reduce the size of ice crystals formed during the churning and freezing process. Smaller ice crystals result in smoother and creamier ice cream. Starting with a chilled base will allow the ice cream maker to freeze the ice cream faster. And the faster the ice cream is frozen, the smaller the ice crystals that are formed. This is particularly important when using home ice cream makers which do not have enough cooling power to make the process fast. If you are in a hurry you can chill the ice cream base in an ice water bath but if you have time it may be better to chill it for several hours in the fridge as you will benefit from aging.
Aging
The aging process improves the capability of the ice cream base to hold the air better and form a stable foam during the churning or whipping step. Aging is considered more important for custard-base ice creams made with eggs. The aging process consists of simply letting the ice cream base rest in the fridge for several hours, usually overnight. There are two things that happen during the aging process:
- Crystallization of milk fat globules. During aging, crystals from milk fat start protruding from the surface of the globule. These protrusions or "needles" are very important when incorporating air while churning the ice cream base as you will learn in the next step.
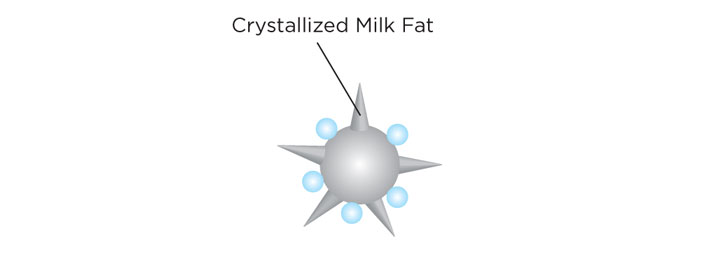
During aging, crystals from milk fat start protruding from the surface of the fat globule
- Low molecular weight emulsifiers present in ice cream from the eggs and/or ice cream stabilizers such as lecithin and mono & diglycerides (glycerin flakes), displace the casein micelle protein that was stabilizing the fat globules against coalescence (from the homogenization phase). As the proteins are displaced from the surface of the fat globules, the crystallized fat "needles" protruding from them get exposed creating free fat globules with "needles".
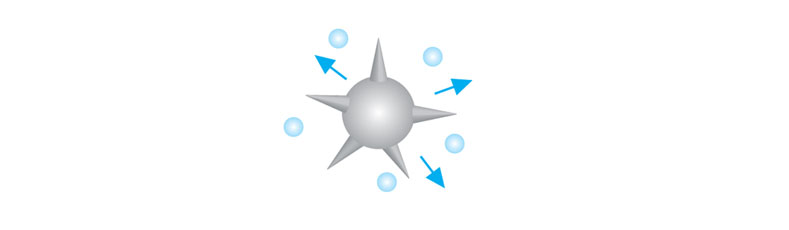
Emulsifiers displace the casein micelle protein that was stabilizing the globules
The side benefit of aging is that you will ensure that the ice cream base is completely chilled and if you are steeping flavors into it, you can continue steeping them while aging to obtain a stronger flavor if desired.
Churning and Freezing
The freezing and churning step is the process of churning and incorporating air into the ice cream while freezing it. The faster you freeze the ice cream, the smaller the ice crystals that are formed and the smoother and creamier the ice cream you will obtain. This is why it is important to start with a chilled base and to have a good ice cream maker that can cool fast. The objective is to reduce the the temperature of the chilled ice cream base from about 5 °C (41 °F) to between -5 °C and -7 °C (23 °F and 19.4 °F). If making ice cream at home, you don't need to spend hundreds of dollars in a good ice cream maker, we'll show you how to make ice cream with dry ice for a fast and cheap cooling that will result in creamier ice cream.
Don't try cooling churning ice cream below -5 °C and -7 °C, it won't happen. At this temperature the ice cream is in an equilibrium. As the ice cream cools down and ice crystals form, the remaining liquid water becomes increasingly more saturated with sugars and viscous, requiring more and more energy to keep it stirred. At this temperature, the amount of energy introduced by the churning process equals the amount of energy being taken out by refrigeration.
The churning process incorporates the air and allows the ice cream base to cool down evenly and fast by exposing the base to the cooling wall. The faster the churning, the more air that is incorporated in the ice cream and therefore produces a higher overrun. Gelato is usually churned at a lower speed than ice cream to incorporate less air and obtain a creamier texture. This is when the stable oil in water emulsion gets converted into a stable foam. But what makes the foam stable? Remember those crystallized fat "needles" protruding from the fat globules?
During the churning process, the fat in the ice cream base gets restructured to make the foam stable. The stabilization of the foam happens by a process called partial coalescence. During churning, the milk fat globules with the crystallized fat “needles” collide, piercing each other and forming a network of partially coalesced fat globules that surround the air bubbles. This new network holds the air being incorporated in the ice cream base and makes the foam stable.
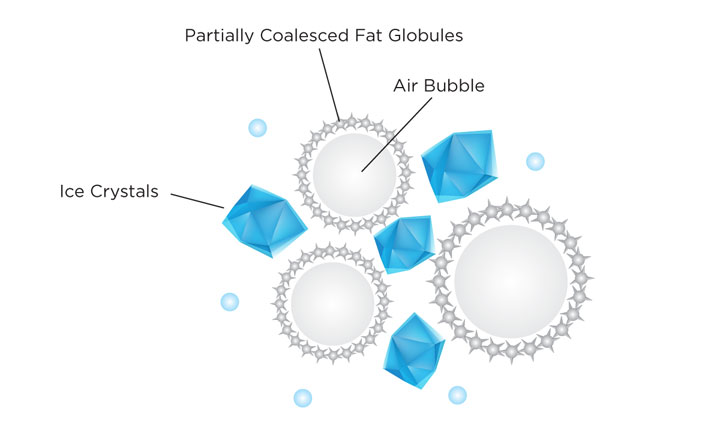
During churning, milk fat globules form a chain around air bubbles making the foam stable
The freezing stage is also when you add the other solids, like chocolate chips, nuts, etc. These should be chilled before incorporating into the ice cream to reduce the formation of large ice crystals and to prevent the ingredients from becoming soggy.
Hardening
Ice cream that comes from an ice cream maker is usually very soft and needs to be frozen further. During churning and freezing only 40% to 50% of the water in the ice cream base gets converted into ice. The hardening step allows the ice cream to freeze further creating a denser and more stable finished product. The ideal temperature to store and harden ice cream is below -35 ° C (-31 °F). Most common freezers won't reach such low temperature but the faster you chill the ice cream, the smaller the ice crystals formed and the smoother and creamier it will be. Use small containers with large surface area, place them at the bottom of the freezer, do not overload the freezer, minimize opening the door and use the fast freeze button if available to accelerate the freezing.
Why is -35 ° C (-31 °F) the ideal temperature to harden and store ice cream? Above this temperature, the small ice crystals in ice cream will start to grow and produce an icier and grainy texture. Below -35 ° C (-31 °F), the remaining liquid syrup from the churning process will turn into glass, permanently trapping the ice crystals, air bubbles and fat globules. Even though both are solids, glass and crystals are different. Crystals are a highly ordered system in which molecules are arranged in particular ways. Glasses are solids with molecules that don't move around but they are highly disorganized as in a liquid. When cooling the viscous syrup below this temperature, the molecules have so little energy available that they can't move around enough to rearrange themselves into ordered crystals.
Resources:
- Cesar Vega, Masters in ice cream research and a PhD in dairy emulsions, has done extensive research on ice cream. He obtained an internship at Heston Blumenthal’s restaurant The Fat Duck in England and is the Co-editor & author of The Kitchen as Laboratory
-The Fat Duck Cookbook
-On Food and Cooking by Harold McGee





